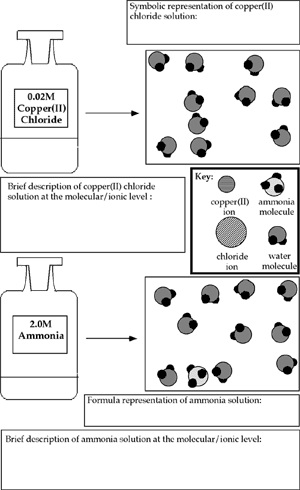
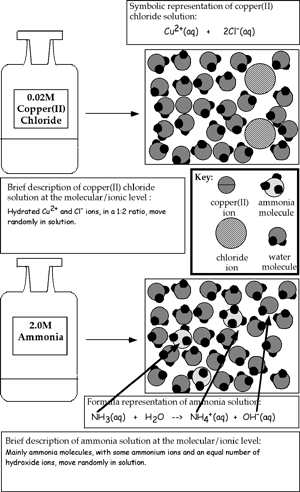
| |
DISCIPLINE
Chemistry
DURATION
One learning session (e.g. small period of contiguous time
in a lecture or tutorial, one week of the subject/unit/course).
However, to be effective, the learning design should be repeated
a number of times, in a number of contexts, throughout the
duration of a subject; that is, whenever a connection between
the lab-, molecular-, and symbolic-levels is useful to understand
a concept. For example, first when explaining acid dissociation,
then later when explaining the difference between acid concentration
and acid strength.
ICT USED
The VisChem animations are available in CD, Web-deliverable
and videotape formats, as commercial products, supplements
to textbooks, and freely-accessible web sites.
Some sources are listed below.
- The resource CD containing all 82 animations in cross-platform
QuickTime format (288Mb), cited as:
Tasker R, Bucat R, Sleet R, Chia W, Corrigan D (1997) VisChem
Resources — Learning Chemistry Through Visualisation
of the Molecular Level.
- A series of three videos, with lecturer’s notes
and student activities, depicting chemical substances and
reactions typically covered in an introductory chemistry
course, cited as:
Tasker R, Bucat R, Sleet R, Chia W, Corrigan D (1996, 1997)
The Molecular World of Water (13 min)
- The Molecular World of Reactions in Water
Part 1: Dissolving, Precipitation and Complexation (25 min)
and
Part 2: Ionic Equilibrium, Acid/Base and Oxidation/Reduction
Chemistry (33 min)
DELIVERY CONTEXT
The learning design is currently implemented in a face-to-face
lecture environment, with students able to work in pairs and
with the support of an experienced lecturer to maintain focus
on key features, and facilitate discussion. The passive, transmissive
nature of this mode of delivery is still the most common in
first-year chemistry, but not ideal. However, the evaluation
study we conducted was limited to this learning context.
The interactive software resources cited above use the VisChem
animations in interactive interfaces. However, the all-important
‘constructivist’ approach of the learning design
has not been implemented in these resources. Evaluation of
their effectiveness for learning has not yet been done.
Interactive online delivery of the learning design will be
possible when a molecular construction tool, that provides
feedback on a student’s model before s/he sees the VisChem
representation, can be produced.
TARGET AUDIENCE
Generally, this learning design is targeted at first-year
undergraduates in science-based courses, but it has also been
used extensively with upper-secondary level (years 11 and
12) HSC Chemistry students. Ideally the approach should be
used in middle-school science classes where the fundamental
ideas (and misconceptions) in chemistry are first introduced.
A specific profile of a student cohort involved in our research
study using VisChem resources is as follows:
“Of the 48 students that we have comprehensive information
on, 32 had previously completed chemistry at HSC level (2U
Chemistry, 3U Science or equivalent), 11 had not studied
beyond junior (year 9/10) chemistry, two had studied year
11 chemistry only, one had not studied chemistry for many
years, and one had an unknown background.”
In general, these students would be at the weaker end of
the academic-ability spectrum of first-year chemistry students
in Australia. However, the VisChem animations are available
on the CD supplement to Atkins & Jones, Chemistry: Molecules,
Matter, and Change, 4th ed. used by first-year students at
the University of Technology, Sydney; the University of Wollongong;
the University of Adelaide; Flinders University; and Edith
Cowan University.
The critical point is that our research has shown that unless
students are convinced that developing accurate mental models
of the molecular world will help them to get higher marks
in the subject’s assessment, they will not engage with
the learning design in a thoughtful way. This is particularly
true with high-achievers with a surface approach to learning.
This condition does require the subject coordinator to assess
the accuracy of student mental models of the molecular level.
This is a challenge because students can often perform well
in conventional exam questions where only rote-learning and
an algorithmic problem-solving approach is needed
COHORT
The nature of the learning design is that there is no limitation
on the number of students involved. In our evaluation study,
the activity involved 90 students in a lecture-theatre setting,
of which only 48 students were studied in detail.
The most important criterion is that the student can obtain
feedback on his/her molecular-level representation before
seeing the VisChem animation. This is essential in order to
focus attention on the key features of the representation.
This feedback can be provided by one or more peers, a lecturer,
or from an ICT resource.
BROADER CONTEXT
A deep understanding of chemistry requires well-developed
mental models of the structures and processes that occur in
the molecular world. The VisChem resources are designed to
help students develop useful models that they can apply to
fundamental concepts in first year, and complex chemistry
concepts in later years.
For example, in second and third year, animations are shown
in advanced contexts to draw out new features to explain advanced
concepts. Furthermore, as students refine their molecular-level
models they can begin to see the limitations of the animations
in portraying some advanced features of the molecular world.
We see this approach as most appropriate when introducing
students to the structure and bonding in common substances,
and to common types of reaction, some examples of topics where
this learning design could be useful later on are:
- Concentration of ions in solution (Note: VisChem animations
of solutions are designed to portray 1M concentrations).
- Thermochemical cycles (e.g. the competition between lattice
enthalpy and hydration enthalpy in dissolution of an ionic
salt).
- Nature of solute/solvent interactions in colligative properties.
- Importance of solvent and temperature in reaction mechanisms.
- Effects of changes on reactions at equilibrium.
- Difference between acid strength and acid concentration.
|